Exploring Iridescence in Ruby-throated Hummingbirds - 29 Jan 2017
[I'm dedicating this blog post to Robert "Dr. Bob" Setzer, who graciously sent me a book entitled, "Evenings at the Microscope" by Philip Henry Gosse, D. Appleton and Company, New York, 1883. Flipping through this volume inspired me to finally write up some personal research I conducted at BASF back in 2012. Please note that this work has not been peer-reviewed. Therefore, any information therein may be construed as Alternative Fact...]
So, here's the backstory. I had come across a blog post by David Sibley discussing why some Ruby-throated Hummingbirds (Archilochus colubris) appear to have orange gorget feathers. The discussion included references to "plates" and "air bubbles" in the barbules of the hummingbird's feathers, and possible causes for the dilution of color from ruby-red to orange. This also came on the heels of me collecting a female Ruby-throated Hummingbird carcass that was found by building maintenance that very morning - I would tag the bird and later give it to a local metropark for their collection.
Since I did not understand a bit of the discussion regarding plates and air bubbles in the feathers, I remember asking the question, "So, which is it? Plates or air bubbles". As a Sr. Research Microscopist at BASF I decided to do a bit of researching to learn more about hummingbird iridescence. A quick Google search produced limited results, but the Hummingbird Website produced a pretty concise explanation. In short, air-filled platelets in the barbules of the gorget feathers act a diffraction gradient to scatter light at specific angles and wavelengths to produce the intense color that ranges from red to brown to black. Greenwalt (1960) summarized it best:
“ Nature varies the iridescent colors of hummingbirds by varying the thickness of the platelets and their air content. Melanin reinforcements in the air gaps create the continuous RI variations that lead to pure and stable color formation. Stacking increases color brilliance”
A more thorough search produced some surprising results. It turns out that Isaac Newton (1704) correctly predicted that iridescence is caused by interference or the presence of thin films in the feathers. Michelson (1911) suggested 'selected reflection' or surface colors seen in reflection on metals or an organic material of high specific absorption. Lord Rayleigh (1919) believed in Newton's Rings or interference coloration and postulated that periodic structures in individual feathers were present. Bancroft, Chamot, and Merritt (1922) discovered plate-like structures in the barbules of individual feathers: broad, flattened and segmented. Since boiling in organic solvents failed to produce color, pigments were ruled out. They also discovered that the angle of incidence is important: barbules in the gorget feathers are angled toward the head instead of the plane of the feather (~45 degrees). Turns out that Rayleigh was right, and Michelson was wrong...
Schmidt (1952) used Hi-Resolution LM to describe a mosaic of plate-like structures of melanin surrounded by a skin of keratin. Greenwalt (1960) would then use spectral reflectance and electron microscopy to verify the presence of stacks of platelets filled w/ air gaps. He concluded that iridescence of hummingbird feathers can be attributed quantitatively to interference of light passing through and being reflected back through these structures, which measure 2-3 microns long, 1-1.5 microns wide, and 100-200 nanometers thick.
 |
| Greenwalt, 1960 |
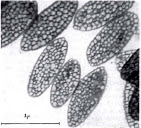 |
| Greenwalt, 1960 |
Having some time, I decided to collect a loose back feather from the dead hummer and see what I could learn using my light microscopes (LM), scanning electron microscope (SEM) and atomic force microscope (AFM) that I have access to in the lab. I was specifically interested to see if AFM could produce some new information (see below).
Examination of the female Ruby-throated Hummingbird reveals iridescent green back feathers. The outer feathers appear iridescent while inner feathers do not. These feathers are visually different from tail or flight feathers that are colored but not iridescent.
I removed a single loose feather and examined it under the light microscope using both reflected and transmitted light. The feather consists of a central shaft with barbs radiating outward along its length. Attached to the barbs are individual barbules that provide the iridescence. Note that not all barbules are iridescent: those near the base of the shaft are colorless or transparent much like the inner back feathers.
Closer examination of the barbules show that they are connected to each other by velcro-like structures called barbicels.
Under the scanning electron microscope the barbicel structures can be seen, as well as the individual barbules attached to the central barb. On this particular sample I noticed residue on the barbules that could be dander, or possibly feather mite eggs, I dunno... I false-colored the SEM images to show the green coloration of the barbules in question. Note the angle of the barbules w/r to the barb; they are oriented open-faced at ~45 degrees to their counterpart on the other side of the barb, and are slightly turned inward as you move toward the tip of the barb.
AFM
Next, I turned to the atomic force microscope (AFM). AFM was first developed in the early 80's by Binnig and Quate. Also called scanning probe microscopy (SPM) the technique utilizes a thin, springboard probe with a pyramid-shaped silicon tip that has a tip diameter of only a few nanometers (imagine a record-player stylus with a diamond tip but shrunk to microscopic size). A laser is bounced off the thin ceramic springboard onto a quadrant photodiode that monitors tip deflection as the probe is scanned across the sample surface (much like braille). We call it atomic force microscopy because the tip deflection is sensitive to the pico-newton attractive or repulsive forces that occur as two surfaces approach each other. A feedback loop ensures that the tip and surface maintain a constant deflection so that large protrusions don't break the tip. This link demonstrates how the technique works.
If we now vibrate the probe at its resonance frequency (called TappingMode™or Phase-imaging), then we can 'tap' the surface as we scan the sample. This does 2 things: 1) it reduces tip-surface interactions that can cause the probe to stick-or-slip during scanning, such as adsorbed moisture or sticky residue, and 2) it can generate information about the viscoelastic behavior of the sample. Because the probe is vibrated with a set frequency and amplitude as it taps the surfaces, the "response" frequency and amplitude for an ideally-hard surface (diamond, for example) would be the same amplitude and phase. However, when the probe contacts a softer surface, the amplitude response is dampened, and there is a corresponding delay in the frequency response (or phase-shift). By monitoring the shift in phase we can generate high-resolution "Phase" images that provide information about the hard-soft properties of a material: harder materials appear brighter and softer materials appear darker.
In this following example of a polymer blend, the Height (topography) image provides little information since it was microtomed flat. The Phase (viscoelasticity) image, however, shows a blend of soft (dark) and hard (bright) polymer domains. The top image has a hard matrix, while the lower image shows a soft matrix.
Green back feather
So, getting back to our iridescent green barbule of the female hummingbird, by placing the AFM probe on the open face of an individual barbule the TappingMode™Height and Phase images reveal several interesting features:
The 5µmX5µm Height image on the left shows platelets just below the surface layer of keratin. The Phase image on the right shows that the keratin has a lamellar structure that runs parallel to the orientation of the platelets (or the length of the barbule). Note, however, in the lower right corner the tiny platelet that is oriented sideways! This indicates that the platelets are not fixed in space, and may float inside the barbule. This is perhaps the first AFM image taken of a hummingbird barbule and shows that the keratin skin layer is not smooth. Does the keratin layer contribute to coloration?
Here is another scan of a green barbule surface. In this case the orientation of the platelets are off plane w/ the keratin's lamellar direction. Several platelets are almost perpendicular in orientation.
Here are 3-D Height images of the barbules w/ and w/o the melanin platelets.
The next challenge involves trying to get Cross-sections of individual barbules and platelets. I took several barbules and embedded them in clear nail polish. After the polish hardened I ultramicrotomed the block with a diamond knife to produce a 50 nm smooth block face for additional AFM imaging. Here is the microtomed block face under reflected light compared to a top view image. You can see the curved arrangement of individual barbules on edge.
TappingMode™ AFM images of the microtomed block face are shown below. Individual barbules show up to 6 layers of platelets with each platelet consisting of multi-celled chambers. An artifact of microtoming is residue collecting inside the chambers - I could not find a way around it.
Curiously, several of the larger melanin platelets appear to contain 2 layers of hollow chambers. Also note the distance between the outside keratin layer and first layer of platelets is ~100 nm.
Gorget feather
Examination of the gorget, or throat feather of the male Ruby-throated Hummingbird reveals bright red iridescent barbules in the distal (outer) third of the feather. The reverse side of the feather shows no iridescence.
Examination of a single barb reveals a range of clear/transparent barbules transitioning to a thin band of iridescent green barbules transitioning to brilliant iridescent-red barbules. Under the SEM the individual barbules are angled 90 degrees to their adjoining neighbors.
Things get VERY interesting at this point. Notice that as you follow the individual barbules from the clear-to-green-to-red regions the barbule orientation gradually curls inward until the iridescent red region is actually caused by reflectance off the BACK side of the barbules.
 |
| 5µ mX5µ m scan of back side of red barbule |
 |
| 5µmX5µm scan area - note that upper surface of the X-section is the back of the barbule! |
 |
| 2µmX2µm scan area |
Results indicate that the melanin platelets accounting for green coloration are larger than those accounting for red coloration. Platelets accounting for red coloration, however, are thicker, but with smaller cells or air bubbles relative to the green feather platelets. This is consistent with Greenwalt's (1960) observations that the refractive index (RI) of melanin is 2.2 (vs. 1.0 for air). Platelets responsible for red iridescence measured 1.85 while those responsible for green measured 1.7. Thicker melanin shifts RI toward red, while larger bubbles shift coloration toward blue.
So, where does this go to answer the original question of where orange gorget feathers come from? Some possible explanations may include: 1) worn keratin layer? 2) bleaching of keratin by the sun? 3) collapse of platelet chambers w/ time would thicken overall melanin and shift toward yellow? 4) loss of orientation of individual platelets could occur? 5) change in the angle of adjoining barbs of individual feathers? 6) a combination of all or some of these?
I had hoped to get an orange gorget feather, but sadly, reports of orange-throated hummingbirds were not forthcoming. So, the true answer will probably need to come from someone a bit more knowledgeable than me. Still, this was a fun and challenging project. Unfortunately, work demands have forced this side project to the back burner permanently, so no new information will be coming any time soon.
I'll finish this off with an image of the melanin platelets using an AFM technique called PeakForce™ Quantitative Nano-Mechanical (QNM) imaging. This technique allows an operator to use a calibrated AFM probe to quantitatively map mechanical properties, such as Young's Modulus (stiffness) or Adhesion, in real time. The following image is a Modulus map of the red gorget feather barbule showing individual melanin platelets against the keratin matrix. The bright contrast of the melanin platelets is due to their increase modulus relative to the softer barbule matrix. The bright edges of the voids in individual platelets are caused by the side of the probe contacting the chamber walls, thus increasing the effective tip radius and thus creating a false-increase in modulus.
 |
| Uncalibrated PeakForce™QNM™ Modulus map |
Acknowledgements
I wish to thank Janet Hinshaw and the University of Michigan's Museum of Natural History for the red gorget feathers. Also to Sherri Williamson (pers. comm.) and Allen Chartier (pers. comm.). And, especially to BASF Corporation for use of their laboratory equipment.
References:
Bancroft, W.D., Journal of
Industrial and Engineering Chemistry, (1922), Vol. 14, No. 10, pp. 808-809,
Doucet, S.M., Shawkey,
M.D., Hill, G.E., Montgomerie, R.,
Iridescent plumage in satin bowerbirds: structure, mechanisms and nanostructural
predictors of individual variation in colour,
2006, The
Journal of Experimental Biology,
209, 380-390.
Greenwalt, C.H., Brandt, W., Friel,
D.D., Iridescent colors of Hummingbird Feathers, Journal of the Optical Society of
America, (1960)
Vol. 50, No. 10, 1005-1013. 3
Johnsgard, Paul A., The
Hummingbirds of North America (1997), 2nd
ed., published by Smithsonian Institution Press in Washington, DC.)
Maia, R., Caetano, J.V.O., Ba ́o,
S.N., Macedo, R.H.,Iridescent
structural colour production in male blue-black grassquit
feather barbules: the role of keratin and melanin, J. R. Soc. Interface (2009) 6,
S203–S211
Michelson, A.A., Phil. Mag. 21, 554
(1911)
Newton, I. Treatise on Opticks,
(1704), London, Vol. 2, p. 55.
Rayleigh, L., Phil. Mag. 37, 98 (1919)
Schmidt, W.J., Z. Naturforsch.
3b, 55 (1948); Naturwissenschaften 14, 313 (1952)
Williamson,
S. http://fieldguidetohummingbirds.wordpress.com/2011/08/15/orange-throated-hummingbirds-not-so-mysterious-after-all/

























Holy cow Jerry. You are a master of the image! I haven't read the whole thing (but I will); but maybe could give an ornithological presentation and/or try to get this published.
ReplyDelete1) I am very honored that you dedicated this post to me. I had the feeling that you would appreciate the book. I knew you did optical stuff at work, and now know a bit more about the tools available. Thanks for the shout-out!!!
ReplyDelete2) Back in "my day" I used optical (phase contrast, Nomarski, etc), TEM and SEM to study the sex lives of seaweeds but the atomic force microscope (AFM) was not yet invented. And I am sure it would have never been available to me anyway!
3) Fascinating study! What a contrast to your digiscoping pics we are used to seeing!Zoom out; zoom in. Different info and perspective. Gets the mind wondering and wanting more!
4) Scholarly! Impressive! I hope you intend to publish this!
5) Thanks for the dedication and a great mind-expanding read!
"Dr. Bob" Setzer
This is a great blog. Recently I have been reading about hummingbird iridescence, including the classic paper of Greenwalt et al (1960) that you reference, where stacks of platelets were investigated using electron microscopy. Although these authors concluded that the reflected color (hue) could be accounted for by the thickness of the platelets and the average refractive index (which depended on the melanin/air proportion) the evidence, as they presented it, is rather obscure. For example i) they use different hummingbird species to illustrate the electron microscopy and reflectance spectra ii) For Anna's hummingbird, which is one of the species they model using thin-film reflection theory, they use an average refractive index of 1.7 (their Table II) yet they show (their Figure 4) that red feathers have a measure refractive index of 1.85. iii) the cross section though a barbule (their Figure 6) from Clytolaema rubricauda (Braziliam ruby) shows a 14 layers meausuring 2.04 um, which give an average platelet thickness of 145 nm. According to their formula that the maximum reflected wavelength = 2 * refractive index * platelet thickness, this would correspond to 435 to 536 nm (depending on refactive index) which is in the blue to green region - but the gorget from this species is red. I haven't found any quantitative data on hummingbird feathers since the 1960 paper until finding your blog. Do you intend to publish this formally? Your mean platelet thickness look consistent with the expected color i.e. green feathers = 2 * 1.7 * 167 = 568 nm while red feathers give 2 * 1.85 * 182 = 673 nm. How did you measure mean platelet thickness? Do you allow for the intervening keratin layers (is that what you mean by plate to plate distance?). Your 5 x 5 um AFM scan shows 12 platelet layers over about 3 um i.e. platelet thickness of 250 nm (including any keratin) i.e. within the range you provide (118 - 304 nm). Did you try using Fast Fourier Transform analysis (Prum and Torres(2003) INTEGR. COMP. BIOL., 43:591–602). I attempted to analyze your 5 x 5 AFM scan using ImageJ and confirmed a repeat spacing of around 245 nm. The range in platelet thickness looks fairly large and would give a corresponding wide range of colors. Is there a problem with distortion of the sample during embedding and sectioning? Clive Bagshaw
ReplyDelete"the true answer will probably need to come from someone a bit more knowledgeable than me" ... Jaw dropping article - thank you (and BASF)
ReplyDelete
ReplyDeleteHummingbirds are the smallest bird that belongs from America. The name of the bird is because of its wings flapping speed that produces the humming sound. It is about 50 to 80 times per second.
Hummingbirds Wallpapers Images
I love hummers, but I have a problem with one. I have a double hanging feeder that one male sits on and will not let other ones feed from either one. Is there something I can do to stop him from chasing the others away?
ReplyDeleteHello,
ReplyDeleteI've just tried to google what exactly makes the beautiful color in the gorgets of the hummingbirds that visit our feeder.
I've just started studying Biology and I was wondering if we could say that the platelets have a crystalline structure? That is what gives chameleons some of their changeable colors...
And to Ron Walker:
I'm not a specialist, but from reading and observing I learned that hummingbirds can be very territorial birds! Cute, but agressive :)
Hello,
ReplyDeleteI've just tried to google what exactly makes the beautiful color in the gorgets of the hummingbirds that visit our feeder.
I've just started studying Biology and I was wondering if we could say that the platelets have a crystalline structure? That is what gives chameleons some of their changeable colors...
And to Ron Walker:
I'm not a specialist, but from reading and observing I learned that hummingbirds can be very territorial birds! Cute, but agressive :)
Hello,
ReplyDeleteI've just tried to google what exactly makes the beautiful color in the gorgets of the hummingbirds that visit our feeder.
I've just started studying Biology and I was wondering if we could say that the platelets have a crystalline structure? That is what gives chameleons some of their changeable colors...
And to Ron Walker:
I'm not a specialist, but from reading and observing I learned that hummingbirds can be very territorial birds! Cute, but agressive :)
I live in upstate NY and have experienced that same problem. To stop this, I moved one feeder out of sight of the other. One feeder hangs on the north side of my deck and the other the west. It didn't take long for the birds to find the moved feeder - about five minutes! Now they can both be used in peace! Hope that helps.
ReplyDelete